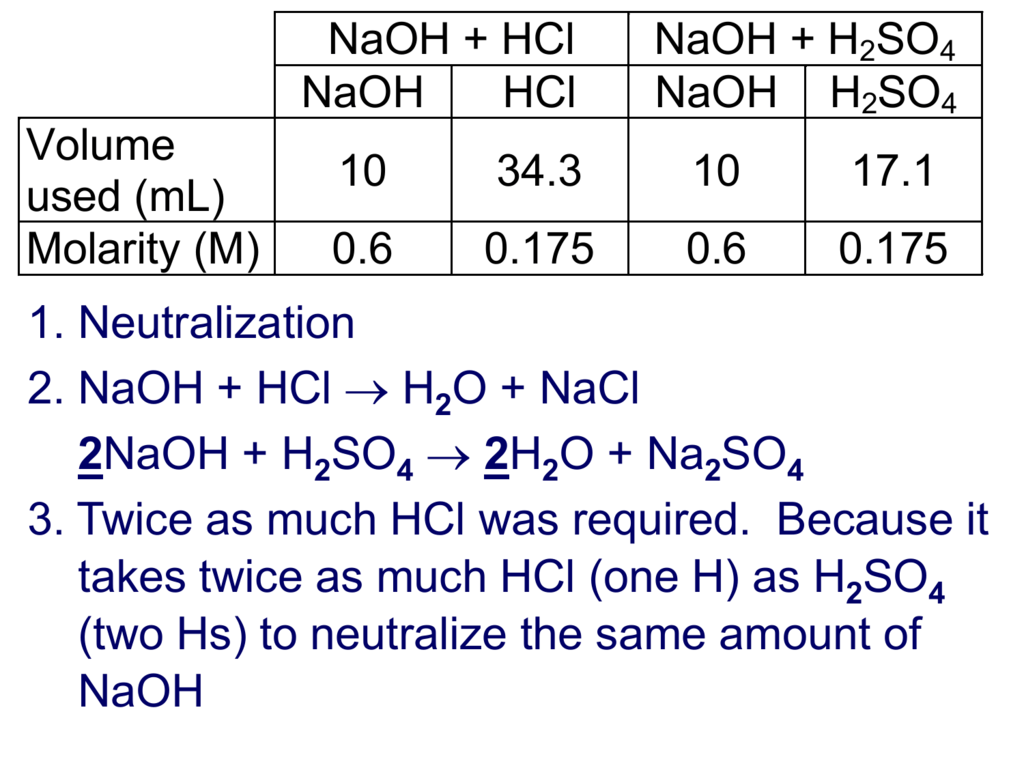
Hcl Titration Formula . two moles of hcl are required to completely neutralize one mole of ba (oh) 2. The strong acid, hcl, furnishes 1 h + ion; in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. in the first standardization the molarity of a sodium hydroxide solution (naoh) will be determined by titrating a sample of. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.
from mungfali.com
The strong acid, hcl, furnishes 1 h + ion; For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. two moles of hcl are required to completely neutralize one mole of ba (oh) 2. in the first standardization the molarity of a sodium hydroxide solution (naoh) will be determined by titrating a sample of.
HCl NaOH Titration
Hcl Titration Formula the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. in the first standardization the molarity of a sodium hydroxide solution (naoh) will be determined by titrating a sample of. two moles of hcl are required to completely neutralize one mole of ba (oh) 2. The strong acid, hcl, furnishes 1 h + ion; a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and.
From www.slideshare.net
Tang 07 titrations Hcl Titration Formula in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the above equation works only for neutralizations in which there is a 1:1 ratio. Hcl Titration Formula.
From cesttiwp.blob.core.windows.net
Titration Volume Formula at Charles Pope blog Hcl Titration Formula two moles of hcl are required to completely neutralize one mole of ba (oh) 2. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The strong acid, hcl, furnishes 1 h + ion; in the first standardization the molarity of a sodium hydroxide solution (naoh) will. Hcl Titration Formula.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Hcl Titration Formula two moles of hcl are required to completely neutralize one mole of ba (oh) 2. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The strong. Hcl Titration Formula.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download Hcl Titration Formula a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. two moles of hcl are required to completely neutralize one mole of ba (oh) 2. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. in a titration, 25.00 cm 3 of 0.200 mol/dm. Hcl Titration Formula.
From vdocuments.mx
Acid and Base Titrations MhChemPage I38 / Acid and Base Titrations Hcl Titration Formula in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. two moles of hcl are required to completely neutralize one mole of ba (oh) 2. a titration is a laboratory technique. Hcl Titration Formula.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Hcl Titration Formula For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. The strong. Hcl Titration Formula.
From www.gulfsouthsolar.com
Extraordinario Solenoide Formación titration of sodium hydroxide with Hcl Titration Formula in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the above equation works only for neutralizations in which there is a 1:1 ratio. Hcl Titration Formula.
From byjus.com
in the titration of Na2CO3 by HCl using methyl orange indicator Hcl Titration Formula in the first standardization the molarity of a sodium hydroxide solution (naoh) will be determined by titrating a sample of. The strong acid, hcl, furnishes 1 h + ion; a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. two moles of hcl are required to completely. Hcl Titration Formula.
From www.scribd.com
Titrations Calculations Questions PDF Sodium Hydroxide Hcl Titration Formula in the first standardization the molarity of a sodium hydroxide solution (naoh) will be determined by titrating a sample of. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. The strong acid, hcl, furnishes. Hcl Titration Formula.
From www.slideserve.com
PPT Acidbase titration PowerPoint Presentation, free download ID Hcl Titration Formula The strong acid, hcl, furnishes 1 h + ion; in the first standardization the molarity of a sodium hydroxide solution (naoh) will be determined by titrating a sample of. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. two moles of hcl are. Hcl Titration Formula.
From ceiuntct.blob.core.windows.net
Hcl And Na2Co3 Titration Calculations at Dorothy Ducharme blog Hcl Titration Formula For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. two moles of hcl are required to completely neutralize one mole of ba (oh) 2. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in the first standardization the molarity of a sodium. Hcl Titration Formula.
From mungfali.com
HCl NaOH Titration Hcl Titration Formula a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. in the first standardization the molarity of a sodium hydroxide solution (naoh) will be determined by titrating a sample of. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid. Hcl Titration Formula.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Hcl Titration Formula The strong acid, hcl, furnishes 1 h + ion; in the first standardization the molarity of a sodium hydroxide solution (naoh) will be determined by titrating a sample of. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. the above equation works only. Hcl Titration Formula.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Hcl Titration Formula The strong acid, hcl, furnishes 1 h + ion; in the first standardization the molarity of a sodium hydroxide solution (naoh) will be determined by titrating a sample of. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. a titration is a laboratory. Hcl Titration Formula.
From www.scribd.com
Cce48Titration of Sodium Hydroxide With Hydrochloric Acid Sodium Hcl Titration Formula the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. a. Hcl Titration Formula.
From www.thinkswap.com
Titration of Sodium Hydroxide with Hydrochloric acid FSC107 General Hcl Titration Formula in the first standardization the molarity of a sodium hydroxide solution (naoh) will be determined by titrating a sample of. The strong acid, hcl, furnishes 1 h + ion; a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. two moles of hcl are required to completely. Hcl Titration Formula.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Hcl Titration Formula two moles of hcl are required to completely neutralize one mole of ba (oh) 2. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is a laboratory technique used to precisely. Hcl Titration Formula.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Hcl Titration Formula The strong acid, hcl, furnishes 1 h + ion; a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in a titration, 25.00 cm 3 of 0.200. Hcl Titration Formula.